February 5, 2025
Rovers Medical Devices announces the introduction of HPV Self-Collection Services in the U.S. by the world’s No. 1 hospital, Mayo Clinic using Evalyn® Brush
Rovers Medical Devices is pleased to share the announcement by Mayo Clinic, Rochester (USA) about the introduction of Human Papillomavirus (HPV) self-collection services utilizing the Evalyn® Brush, a state-of-the-art device designed to empower women with a convenient and effective method for Cervical Cancer screening.
Mayo Clinic, named the No. 1 hospital in Newsweek’s 2024 list of the “World’s Best Hospitals” for the 6th straight year, announced that: “Mayo Clinic is one of a few medical organizations in the U.S. that will provide eligible patients with the option of an FDA-approved self-collection HPV test. The alternative screening is expected to become available at Mayo Clinic practices in the Midwest beginning February 5 and then later expand to Mayo Clinic in Arizona and Mayo Clinic in Florida“. The announcement further quotes Dr. Kathy McLaughlin, family physician specializing in Cervical Cancer prevention: “We’re calling it the HPV self-collection test. The difference is that instead of your clinician putting in a speculum and doing a swab of the cervix, the patient would use a self-collection device to get a vaginal sample to test for HPV” (Courtesy: Mayo Clinic News Network)
More information about the HPV self-collection services and the Mayo Clinic, can be found on Mayo Clinic News Network. (YouTube: https://www.youtube.com/watch?v=6GqjGLcmszM&t=6s)
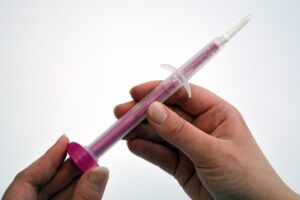
Left image: Rovers Evalyn brush / Right image: Mayo Clinic Rochester (courtesy Rovers Medical Devices)
May 17, 2024
Rovers Medical Devices received the EU Medical Device Regulation (MDR) certification
Proudly sharing that Rovers Medical Devices has received the EU Medical Device Regulation (MDR) certification, granted by the esteemed Notified Body of TüV NORD CERT GmbH (0044).
This certification marks a significant milestone, showcasing our unwavering commitment to excellence and compliance with European market regulations.
The EU MDR certification underscores our dedication to delivering innovative and high-quality medical devices, specifically designed for cancer screening, that meet the most stringent regulatory standards. We are thrilled to continue our mission to contribute to the prevention of cancer on a global scale.
For more details and to view our MDR certificate, please visit the Quality section on our website, or follow the link https://www.roversmedicaldevices.com/quality-certificates/
May 14, 2024
Milestone in Women’s Health: FDA Approves Self-Collection for HPV Testing
We are thrilled to share a groundbreaking advancement in women’s health in the United States. The FDA has approved self-collection solutions for human papillomavirus (HPV) testing, aimed at improving access and participation in cervical cancer screening. With this approval, the use of our Evalyn® Brush can significantly contribute to ending cervical cancer!
“This self-collection method can be performed in various healthcare settings, including non-traditional locations like retail pharmacies and mobile clinics. Women can collect a clinically-validated vaginal sample using our Evalyn® Brush, which is then sent to a lab where it can be analyzed with e.g. the Roche cobas® HPV test, which is already covered by private insurance, Medicare, and Medicaid.”
Key Facts:
- 50% of cervical cancer patients in the USA have never been screened.
- The American Cancer Society estimates that in 2024, there will be about 13,820 new cases of invasive cervical cancer and about 4,360 deaths from cervical cancer.
The Evalyn® Brush is the most reliable self-sampling device for HPV screening. Here’s why:
- Reliability: It provides results as accurate as clinician-collected samples.
- Consistency: The sample is consistent and uniform across different populations.
- Stability: The sample remains stable, ensuring valid results over time and under various temperature conditions.
- Patient-Friendly: It is easy and comfortable for women to use.
We are more than happy to answer any questions you may have about our Evalyn® Brush and its benefits.
For more information, please visit: Evalyn® Brush Product Page
Reference: FDA Approval Announcement
March 4, 2024
Rovers Medical Devices now part of Halma
Today we are proud to announce that Rovers Medical Devices (“Rovers”) has become part of the Halma Plc. (“Halma”) group of companies, after a transfer of ownership from its current shareholders a.o. Smile Invest to Halma.
Halma is a global group of life-saving technology companies, focused on growing a safer, cleaner, healthier future for everyone, every day. Its purpose defines the three broad markets it operates in: Safety, Environment and Health. It employs over 8,000 people in more than 20 countries, with major operations in the UK, Mainland Europe, the USA and Asia Pacific. Halma is listed on the London Stock Exchange (LON: HLMA) and is a constituent of the FTSE 100 index.
Rovers will continue its role as a leading designer and manufacturer of medical devices for cancer prevention and Women’s Health worldwide. The Rovers organisation, facilities, brand and products will all remain “as is” and our Operations, Sales and Customer Service will continue to operate as usual.
This investment by Halma only confirms that Rovers and its products are considered the gold standard in cancer prevention and diagnostics and an acknowledment of the quality of our products and services.
We look forward to sharing more updates as we integrate with Halma and continue to pursue our shared goals.
Thank you for being a part of our journey. Here’s to the next chapter in our story!
March 1, 2024
Rovers Medical Devices at EUROGIN 2024
From March 13-16th, Rovers Medical Devices will display its latest solutions in Cervical Cancer screening during the EUROGIN Congress in Stockholm, Sweden.
EUROGIN is the world’s leading international congress on HPV infections and associated cancers with a focus on prevention and diagnostics. During the congress, Rovers will be present with a booth and its complete portfolio of cancer screening devices, including the world’s most reliable self-sampler for HPV, the Evalyn® Brush. Customers and other Medical Professionals are welcome to visit and learn more about Cervical Cancer prevention from the Rovers team at booth #A03.
November 23, 2023
Rovers Medical Devices received the ISO 13485:2016 (MDSAP) certificate from TUV USA Inc,
Proudly sharing herewith that:
The Certification Body TUV USA Inc, hereby confirms that Rovers Medical Devices B.V. complies to the requirements ISO 13485:2016 (MDSAP).
MDSAP Medical Device Single Audit Program is an international program wherein Rovers satisfies the very stringent regulatory requirements of multiple participating countries, including the United States, Canada, Brazil, Australia, and Japan.
This is an important milestone as Rovers Medical Devices B.V. is globally entrenched for the betterment in Women’s Health.
This Certificate is fundament under all our efforts.
Please find the MDSAP-certificate under: Quality
October 31, 2023
Rovers Medical Devices partners with Healthtracka to Introduce Cutting-Edge HPV Testing Services in Nigeria
Rovers Medical Devices, a global leader in cervical cancer screening devices, and Healthtracka, a fast-growing healthcare diagnostics company from Lagos, are proud to announce their partnership to launch state-of-the-art HPV testing services in Nigeria. This collaboration marks a significant milestone in the fight against cervical cancer in Nigeria, offering women access to advanced diagnostics that can save lives.
Cervical cancer is a major public health concern in Nigeria, with thousands of women affected each year. Human Papillomavirus (HPV) is a common sexually transmitted infection and a primary risk factor for cervical cancer. Early detection of high-risk HPV types is crucial in preventing cervical cancer and Healthtracka’s new self-sampling option aims to make this crucial service more accessible and convenient for Nigerian women. The partnership endorses the World Health Organization (WHO) global initiative to have 70% of women screened using a high-performance test by the age of 35, and again by the age of 45, in order to ultimately eliminate cervical cancer.
Key highlights of this partnership include:
Advanced HPV Testing Services: Healthtracka will be offering the most advanced HPV testing services using the Evalyn® Brush, a trusted and widely used HPV self-sampling device. This technology ensures reliable and comfortable sample collection, enhancing the diagnostic process.
Accessible Testing: Healthtracka and Rovers will make HPV testing services accessible at various healthcare centers across Nigeria. This accessibility aims to reach a larger population, especially in rural areas where healthcare access is limited.
Corporate Programs: Healthtracka offers tailored corporate programs for Nigerian companies who like to take extra care of the well-being of their female staff. Earlier this year, Uber partnered with Healthtracka by providing HPV Self-Sampling Kits which allowed female drivers, who are always on the road, to have the screening done at the most convenient time.
Educational Initiatives: Both organizations are committed to raising awareness about the importance of regular HPV testing as a preventive measure against cervical cancer. Educational campaigns have been launched to inform women about the benefits of early detection and the role HPV plays in cervical cancer.
Ifeoluwa-Dare Johnson, CEO of Healthtracka and recently recognized by the NASDAQ Entrepreneurial Center in the Milestone Maker cohort, commented on the partnership, saying, “We are excited to collaborate with Rovers to provide comprehensive HPV testing services in Nigeria with reliable self-sampling. Cervical cancer is a significant health issue, and we are committed to playing a pivotal role in reducing its prevalence by promoting early detection. With the introduction of our state-of-the-art HPV testing services, we hope to empower women with the knowledge and tools to safeguard their health.”
Rovers Medical Devices, a reputable global leader in cell sampling technology, has expressed its enthusiasm for the collaboration. The company’s CEO, Roel Leenders, stated, “We are delighted to work with Healthtracka in their mission to make HPV testing services readily available in Nigeria. The Rovers Evalyn® Brush is a proven, user-friendly vaginal sampling device that simplifies the screening process. We believe this partnership will make a significant impact in the fight against cervical cancer in Africa.”



October 28, 2021
The Dutch Health Council recommends self-sampling
The Dutch Health Council recommends that self-sampling kits should be offered equivalently to all women who are invited to undergo population screening for cervical cancer, and should automatically be included with these invitations. It takes the view that complete freedom of choice – coupled with the convenience of not having to apply for the set separately – should substantially mitigate any obstacles to participation in the population screening programme. This should enable more cases of cervical cancer to be detected at an early stage.
The Dutch screening organisation is using the Evalyn® brush since 2017.
Click here to read the complete recommendation.
September 10, 2021
Rovers Medical Devices co-operates with BIO Ventures for Global Health to screen women in Rwanda for Cervical Cancer
According to the WHO, nearly 90% of the Cervical Cancer deaths in 2018 occurred in low- and middle-income countries. This is mainly because access to public health services is limited and screening and treatment for the disease have not been widely implemented. Rovers Medical Devices has been selected by BIO Ventures for Global Health (BVGH) to supply screening devices to the Rwanda Biomedical Center (RBC) who will launch a cervical cancer screening campaign in the Bugesera district in the country.
The Rovers Viba-Brush® is a smart and comfortable sampling device that will be used for HPV testing in local laboratories. The campaign of Cervical (and breast) Cancers screening in Bugesera targets women aged 30 to 49 who are encouraged to go for screening in the nearest health centers. The women will be supported by local nurses and localized information and instructions. Rovers and BIO Ventures for Global Health (BVGH) co-operate with the Rwanda Ministry of Health in this important project to improve healthcare services in Africa.
Click here to read the complete article about the screening campaign.

June 30, 2021
Maccabi HMO deploys pilot with the Rovers Evalyn® Brush for women who have not been tested for cervical cancer for more than three years
Israel’s Maccabi HMO is conducting a preliminary pilot with the Rovers Evalyn® brush for women who have not been tested for Cervical Cancer for more than three years.
Although the Ministry of Health recommends that women between the ages of 25 and 65 perform a Pap test once every three years, only about half of these women actually participate.
Now, for the first time in Israel, self-sampling HPV tests were promoted among women who are insured by the HMO and have not been tested for more than three years. The Evalyn® HPV self-sampling test, provided via Rovers Medical Devices local partner Gamidor Diagnostics, could be performed by the women at the health facility or in the comfort of their own home. First results already showed that 7 out of 100 women who participated in the preliminary pilot have been diagnosed positive for high risk Human Papilloma Virus (HPV), the virus type that can cause Cervical Cancer. Without this pilot, there is a possibility that these women would have never been found.
May 28, 2021
Rovers Medical Devices at EUROGIN 2021: HPV Self-sampling
Looking forward to the EUROGIN 2021 Congress where Rovers Medical Devices, will be presenting its latest innovations in HPV screening. Besides a smart sample transport solution for LMIC’s, we will also highlight the Evalyn® Self-sampler. In 2020, HPV Self-sampling has proven to be a valuable tool for the continuation of Cervical Cancer screening programmes during COVID19 lock-down times, when physical visits to the doctor were restricted. The Evalyn® HPV Self-sampler can be used in the comfort of your own home and sent via mail to the physician or laboratory for further analyses. Furthermore, the Evalyn® Brush can also play an important role in re-starting the regular Cervical Cancer screening programs in 2021, without additional pressure on the medical professionals who may cope with backlogs. The EUROGIN will be virtual from 30th May – 1st June.
Oct 23, 2020
MDSAP
Certificate & ISO 13485:2016 as a resultant of a true team effort, issued today by TüV USA certifying that the Rovers Medical Devices Quality Management System is in conformance with ISO 13485:2016 under MDSAP for Medical Device requirements.
Very proud to be able to share this recognition of our undisputed embedded attention to above par Quality, in procedures and products. To the benefit of all stake holders involved, and designed with the end-user and patient in mind.
We Care for The World.
Please find the MDSAP-certificate under: Quality
Feb 13, 2017
New publication in the Int. Journal of Cancer titled: “Human papillomavirus self-sampling for screening non attenders: Opt-in pilot implementation with electronic communication platforms”
In this study the Evalyn device was used with RFID chip for identification.
Jan 14, 2017
HPV self-sampling to become new public cervical cancer screening alternative in the Capital Region of Denmark

HPV self-sampling to cervical screening non-attenders will become a public health care offer in 2017 for women in the Capital Region of Denmark. Initiatives and funding to improve the screening attendance, among them HPV self-sampling, has been described in the “Cancer Act-4” released by the Danish Ministry of Health in October ´16 after receiving recommendations from the Danish Medical Authority in August of the same year.
The Rovers Evalyn device has been chosen for this purpose.
More information can be found on:
Dec 9, 2015 Media Release
Rovers Medical Devices wins the first tender for self-collection sets for hrHVP testing in the new National Cervical Cancer Screening Program in the Netherlands

Rovers Medical Devices is proud to announce that it has been awarded a 5-year contract by the FSB (Collaboration Facility Population Screening) in the Netherlands for the supply of the Rovers® Evalyn® Brush self-collection set for hrHPV testing for non-responders of the national cervical cancer screening program. The decision concludes an extensive public tender process in which providers were assessed on their ability to meet performance, quality and pricing criteria.
In the Netherlands, non-responders of the cervical cancer screening program account for more than 50% of all the cervical cancer cases. It is expected that the possibility for non-responders of the cervical cancer screening program to use a self-collection set for hrHPV testing will lead to a significant reduction in the number of women that will develop cervical cancer.
Announcement Dutch Ministry of Health, Welfare and Sport:
http://www.rivm.nl/
About the Evalyn® Brush from Rovers Medical Devices
The Evalyn® Brush was specifically developed for the purpose of self-sampling at home. In total more than 50000 women have been involved in studies in which the Evalyn Brush was used. The product was validated in studies targeting different population groups: high risk, responders and non-responders of the screening program. The Evalyn® Brush has in-built features to assure correct sample taking by women.
About Rovers Medical Devices
Rovers Medical Devices is based in Oss, the Netherlands. Rovers designs, develops, manufactures and markets cell sampling devices for cytological-, bacteriological-, viral (HPV)-, and DNA testing. Rovers® products are being used in more than 50 countries all over the world.