During the past decades anal cancer incidence has increased in line with the HIV epidemic. The rate of anal cancer in HIV-positive MSM is currently 35 per 100,000 which is approaching the same level as cervical cancer in women.1
HPV anal screening without the need for proctoscope or anoscope
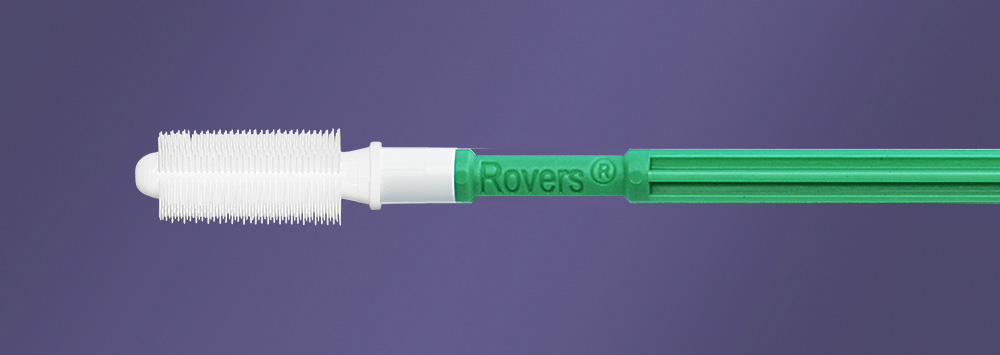
Human papillomavirus (HPV) infection has been implicated as the prerequisite for the development of the majority of anogenital malignancies accounting for more than 90% of the cervical and anal cancers.2 These observations support the need to implement screening to prevent anal cancer in the HIV-infected population. The need for high quality sampling method to allow for testing has led to the development of the Anex® Brush.
The Anex® Brush has been developed in collaboration with physicians by Rovers Medical Devices, specialist in the development of cell sampling devices for over 30 years. The device has been developed to allow for optimal sampling of the anal canal without the need for a proctoscope or anoscope. The entire anal canal is sampled, including the anal transformation zone.
Benefits:
- Sampling of the entire anal canal including the anal-rectal transformation zone.
- Easy to use: No need for a proctoscope or anoscope.
- Single sample that can be used for HPV testing, cytology, and protein testing. HPV detection based on DNA or RNA.